
Thousands of ocean species build protective shells to survive. These organisms create their shells, which can be paper thin, by drawing certain molecules from water around them. The rising acidity is depleting these molecules, forcing these animals to work harder to build their shells, leaving them with less energy to get food or reproduce. Eventually, the organism can't compete ecologically and will die. When acidity becomes too high, shells dissolve. Already, water acidic enough to dissolve shells can be seen off the coast of California. It's thought that water like this will become more and more common. If we continue on the current path, the Southern and Arctic ocean will be so corrosive that it will dissolve all shells by mid-century. Conditions such as these haven't existed since the time of the dinosaurs. By recreating those conditions so quickly, many organisms won't be able to adapt to the changes.
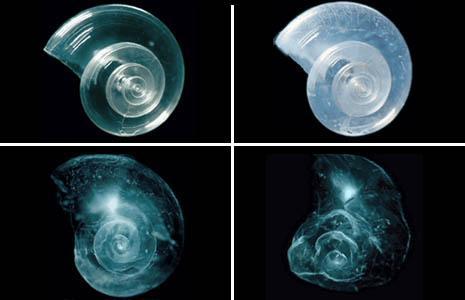
Shell in polar water predicted for 2100
Corals and plankton play a key factor in ocean food webs. Terapods, planktonic snails that live throughout the world's oceans, particularly in arctic waters, are especially vulnerable. Terapods taken from the arctic to be studied have already shown symptoms of shell erosion. There's growing alarm that species such as terapods, a major source of food for fish, will become extinct as the ocean's acidity rises. If the small organisms are effected by ocean acidification, than it ripples across all the other species, causing the larger animals to become even more endangered.

Similarly, ocean acidification has a major impact on human life. 500 million people depend on coral reefs for their daily food and income. As ocean acidity rises, coral reefs and marine life are being threatened. 50% of coral reefs are now gone, disappearing twice as fast as Rainforests.This is happening so fast that it poses a serious threat to biodiversity and marine life. If left unchecked, ocean acidification could destroy all our coral reefs by as early as 2050. It also has the potential to disrupt other ocean ecosystems, fisheries, habitats, and even entire oceanic food chains.

We can slow the rate of ocean acidification through numerous means. Marine protected areas, like national parks in the sea, shelter marine life from industry and development, sustainable fishing practices allow fish stock to regenerate, and keeping the oceans clean and increasing fish populations help it to be more resilient to acidification are all ways to help save the world's oceans. The only question now is, will we?
- Victoria Mehlhaff -
No comments:
Post a Comment